New Drug Designations – November 2023
Shots:
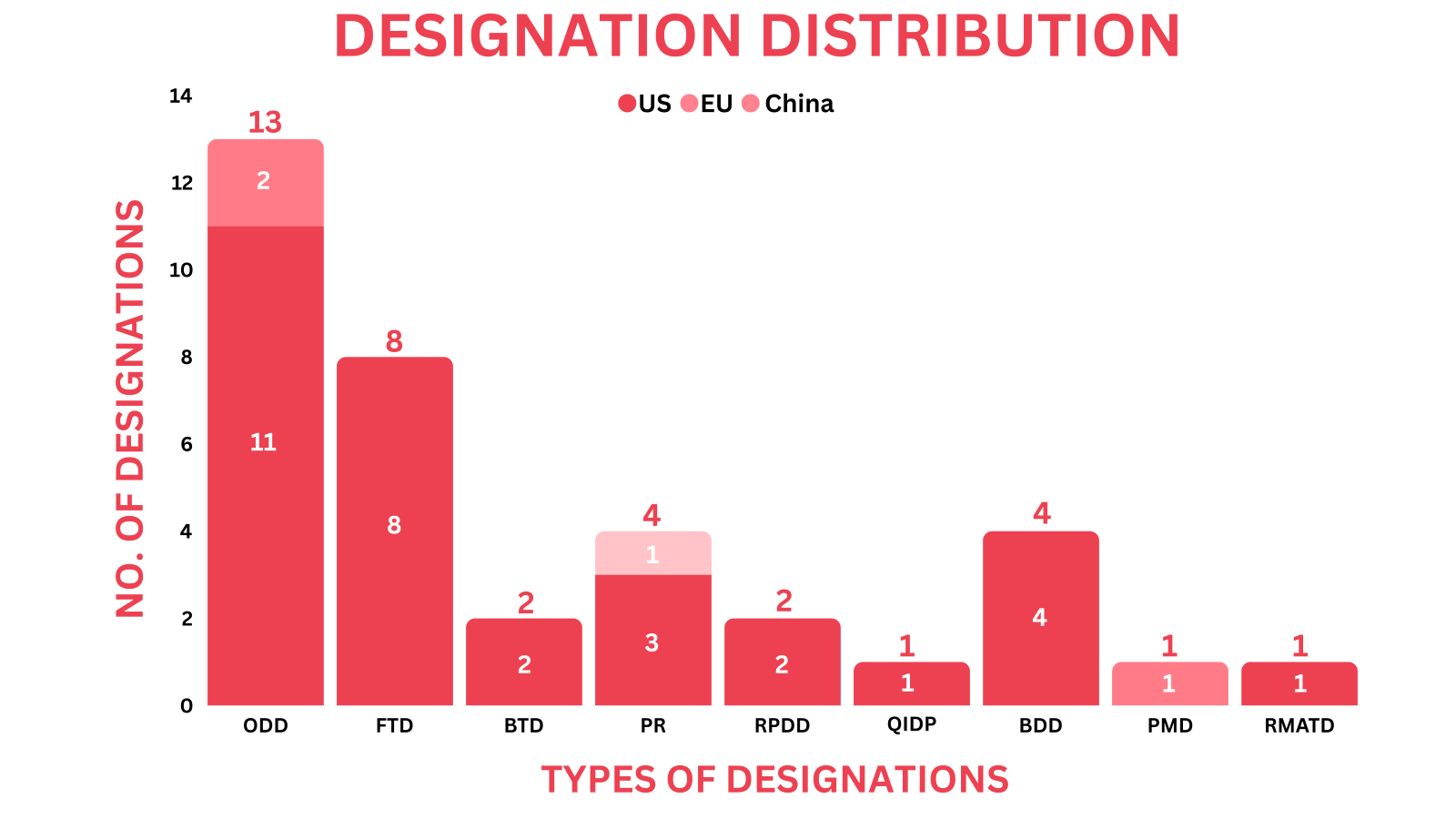
- PharmaShots’ designation report provides a concise overview of several drugs and their designations by the US FDA, EMA and China. This month’s report includes 3 biological drugs, 12 small molecules, 13 cell and gene therapies, 1 peptide, 3 exosome-based therapy and 4 devices
- Atsena Therapeutics’ capsid AAV.SPR gene therapy, focused on the treatment of Leber congenital amaurosis (LCA1) is the drug to receive both RMAT and ODD from the US FDA
- PharmaShots has compiled a list of a total of 32 drugs and 4 devices awarded with designations by multiple regulatory bodies in November 2023

Elsunersen (PRAX-222)

- EMA’s PRIME designation was granted based on the Part 1 data from the EMBRAVE study which demonstrated reduction in seizures and improvement in seizure free day, along with preclinical data
- EMBRAVE is a P-I/II open-label cohort involving pediatric patients aged 2 to 18 yrs. diagnosed with early-onset SCN2A, SCN2A-DEE are consumed over a period of up to 13 wks.
- Elsunersen is an ASO designed to selectively decrease SCN2A gene expression, directly targeting the early-seizure-onset SCN2A-DEE to treat seizures & other symptoms incl. SCN2A mutations
- Elsunersen has received ODD & RPD from the FDA, and ODD from the EMA for the treatment of SCN2A-DEE & the program is ongoing under a collaboration with Ionis Pharmaceutics, Inc. (NASDAQ: IONS), & RogCon, Inc.

Padcev (enfortumab vedotin-ejfv) with Keytruda

- FDA accepted for priority review a sBLA for PADCEV (enfortumab vedotin-ejfv) with KEYTRUDA for 1L locally advanced or metastatic Urothelial Carcinoma patients (la/m UC)
- This sBLA and the priority review is based on data received from P-III EV-302/ KEYNOTE-A39 which showed improved OS and PFS in LA/M UC patients vs pt-CT with no new safety issues
- FDA PDUFA date is May 9, 2024, and the application is reviewed under its RTOR program. If approved, this would be the 1st treatment option for cisplatin eligible & ineligible patients
Prademagene Zamikeracel (pz-cel)

- The US FDA has granted priority review for the BLA for pz-cel, COL7A1 gene for the treatment of patients with RDEB. The US FDA has set a PDUFA date of May 25, 2024
- This BLA is supported by the efficacy and safety data from the pivotal P-III (VIITAL) study and confirmatory evidence from a P-I/IIa study. According to both studies pz-cel on large and chronic wounds resulted in long-lasting healing and pain relief
- The VIITAL study data was presented during the ISID Meeting in May 2023 & Long-term follow-up data (up to 8years) from P-I/IIa study published in Orphanet Journal of Rare Disease
IBI351

- The results from a single-arm, P-II study (NCT05005234) evaluating the safety & efficacy of IBI351 monotx. on NSCLC with KRAS G12C mutation & was intolerant to standard treatment in China. Results were presented during ESMO Asia Congress 2023
- In oral presentation at AACR 2023 (data cut off Feb 10, 2023), Results from P-I study, NSCLC patients (n=67) depicted an ORR of 61.2% & DCR of 92.5%. Better effifcacy was observed in 30 NSCLC patients who received IBI351 (600mg, BID) with ORR 66.7% and DCR 96.7%
- The median PFS was 8.2m, the 6- & 9-month PFS rate were 58.9% and 47.3% respectively, median follow up 8.1mos. As of Nov 2022, no DLT reported and MTD was not reached
- No TRAEs related death or discontinuation reported. Innovent is exploring combination therapy of IBI351 in previously untreated advanced NSCLC patients with KRAS G12C mutation
- Two P-Ib studies of IBI351, in combination with cetuximab (ERBITUX®, EGFR inhibitor) and sintilimab (TYVYT®, PD-1 inhibitor) respectively, are currently ongoing.
Breyanzi (lisocabtagene maraleucel)

- The sBLA & priority review are based on the results from the P-I/II (Transcend CLL 004) the first pivotal trial to show clinical benefit with a CD19-directed CAR T cell therapy in patients with r/r CLL after following treatment with a BTKi & BCL2i
- The application received priority review with an expected PDUFA date of March 14, 2024. The P-II study evaluates Breyanzi at the recommended dose from the P-I monotx. arm
- The EP was CRR incl. complete remission with incomplete bone marrow recovery on guidelines from 2018 iwCLL based independent review committee

OTL-203

- Received FTD based on an ongoing single-center PoC study 8 patients diagnosed with MPS-IH were treated at Ospedale San Raffaele in Milan, Italy with investigational OTL-203 b/w Jul 2018 & Dec 2019 Interim results published in the NEJM further study investigators have observed continued cognitive development & evidence of continued growth within normal range and improvements in skeletal health
- Orchard is planning a registrational P-III study (HURCULES) which is planning to recruit 40 patients (MPS-IH) with a confirmed diagnosis who will be randomized 1:1 to receive either OTL-203 or allogeneic HSCT
- Orchard planning to recruit these patients at 6 sites across US & EU. OTL-203 has previously received RPD & PRIME designations from the FDA and EMA, respectively
Zotatifin

- FTD has been granted based on preclinical and clinical data for Zotatifin (incl. Safety & efficacy data for the ZFA triplet (Zotatifin + Fulvestrant + Abemaciclib)) for 2l/3L ER+/HER2-ve advanced and metastatic breast cancer patients progressed after ERT/ CDK4/6i
- The P-II being investigated in the zotatifin dose escalation and expansion study presently enrolling cohorts for ER+/HER2- breast cancer. Data presented at the ASCO 2023 showed that, in heavily pretreated patients, PR: 5/19 (26%) [4 confirmed & 1 unconfirmed]
- CDK 4/6 & fulvestrant treatments and all five had received one or more prior lines of CT in the metastatic setting. Results based on safety and tolerability to date dose escalation was resumed in combination with fulvestrant (ZF doublet)
- Further updates to interim data from (ZFA triplet) cohort and resumed dose escalation cohorts were presented at SABCS in Dec 2023. Zotatifin is a potent and sequence-selective RNA inhibitor of helicase eIF4A which suppresses the network of cancer driving proteins, incl. Cyclins D and E, CDKs 2, 4 and 6 and select RTKs as well as KRAS
CM-101

- CM-101 is a mAb that neutralizes CCL24, is therapeutic target has been validated in extensive preclinical trials and Chemomab researchers have demonstrated preclinical proof-of-concept in multiple animal and patient sample studies
- CM-101 was safe and well tolerated in P-I and P-II studies to date. CM-101 has ODD from the FDA and Europe’s EMA and is currently being evaluated in a P-II (SPRING) study of the safety and tolerability in PSC patients with expected topline results in H2 2024
BI 764532

- The investigational BI 764532 is T-cell engager that redirects T cells towards cancer cells expressing the DLL3 protein, whose disease has progressed following at least one prior line of treatment incl. platinum-based CT
- OBT & BI partnered in 2013, where BI will used OBT’s proprietary OGAP drug discovery platform for identification of the DLL3 antigen
- For BI 764532, this is the third FTD and received ODD by the FDA for the treatment of SCLC, r/r NEC
RZ-001

- The company P-I/IIa IND approval for RZ-001 from the FDA and South Korean MFDS in GBM and study will investigate the safety, tolerability, and efficacy of RZ-001 in patients with Glioblastoma. RZ-001 will be administered to subjects in accordance with the intended dose escalation design
- RZ-001 targets and cleaves hTERT mRNA and replaces the mRNA with the therapeutic gene RNA. The reprogrammed hTERT mRNA is trans-ligated with an HSVtk-encoding sequence to induce anti-cancer activity and cytotoxic effect
ADP101

- This FTD is supported by the results from P-I/II Harmony trial (NCT04856865) assessing the efficacy and safety of ADP101 to help patients with one or more food allergies become desensitized. Study results presented at the European Academy of Allergy & Clinical Immunology Congress 2023
- The study results showed that ADP101 is dose-dependent with a favorable safety and tolerability in pediatric patients allergic to single or multiple food sources in the product and has shown clinically substantial responses as a multi-OIT food allergy desensitization therapy
AVB-101

- The US FDA has cleared the IND application for AVB-101, has been developed as a possible one-time treatment to stop the progression of the disease by giving the brain regions damaged by Frontotemporal Dementia a functional copy of the GRN gene to restore normal progranulin levels
- The company announced recently that they started enrolling in EU countries for ASPIRE-FTD study to evaluate the safety and efficacy of AVB-101 in patients with FTD-GRN
- The US FDA and EU both granted the ODD to AVB-101 for treatment of FTD in 2022
Sonala-001

- The safety and preliminary efficacy of Sonala-001 SDT are presently being assessed in the P-I/II trial (SDT-201), an open-label drug and energy dose ranging and expansion study
- Sonala-001 + INSIGHTEC Exablate 4000 Type-2 ultrasound device has received FTD for the treatment of patients with DIPG
- In 2021, Sonala-001 has received ODD for malignant gliomas
- Preliminary data from the dose-ranging cohort were presented on Nov 16-19, 2023, SonALAsense-hosted symposium. SNO Annual Meeting in Vancouver

EP0031/A400

- EP0031/A400 is currently being investigated in a global, modular P-I/II trial in patients with advanced RET-altered tumours incl. patients who are naïve to, or have progressed on, first generation SRIs
- Received IND approval from FDA in Jun 2022 based on preclinical results where EP0031/A400 demonstrated favorable inhibition against key RET kinases in-vitro and in-vivo settings with improved penetration of the blood brain barrier vs. first generation SRIs
- Since March 21, Kelun-Biotech granted Ellipses an exclusive license for EP0031/A400 in certain territories incl. the US and EU, with Kelun-Biotech retaining certain rights in Greater China. EP0031 is being developed in partnership with Kelun-Biotech & is also known as A400 in Kelun-Biotech’s pipeline
NT-I7

- CRO under contract to NIAID, part of the NIH, is conducting a study that employs well-developed NIAID ARS rodent models to investigate NT-I7’s efficacy as a potential treatment & NIAID supporting this research through product development funding to the CRO
- NT-I7 exhibits favorable PK/PD and safety profiles & being studied in multiple studies in solid tumors & as a vaccine adjuvant & studies are being planned for testing in hematologic malignancies, additional solid tumors & other immunology-focused indications
XTMAB-16

- XTMAB-16 has received ODD by the EMA. The US FDA previously granted ODD to XTMAB-16 in Nov 2020
- The company announced that the first US patient has been recruited in its P-Ia/IIb global study to evaluate XTMAB-16’s ability to potentially help patients for the treatment of pulmonary sarcoidosis, a chronic, multisystem inflammatory disorder
EPI-321

- EPI-321 has received ODD by the US FDA for the treatment of facioscapulohumeral muscular dystrophy (FSHD). EPI-321 is single dose therapy being developed to inhibits the abnormal expression of the DUX4 gene
- The company plans to initiate a FIH, P-I/II study of EPI-321 in the H1’2024. The study will be designed to assess the safety, activity and preliminary efficacy of EPI-321 with FSHD
ARCT-032

- ARCT-032 has received ODD by the US FDA to treat Cystic Fibrosis (CF). The first patient of P-Ib study completed two administrations of ARCT-032
- The interim P-Ib data is expected in H1’ 2024
- ARCT-032 is developed using Arcturus’ LUNAR lipid-mediated aerosolized platform to deliver CFTR messenger RNA to the lungs
- The ARCT-032 program is supported by preclinical data in rodents, ferrets and primates, which showed restoration of CFTR expression and function in human bronchial epithelial cells
LP-284

- ODD received for the treatment of HGBCL with MYC & BCL2 rearrangements
- The company has initiated P-I FIH study for LP-284 in B-cell NHL incl. HGBL and MCL.This ODD marks as the 2nd ODD for LP-284 where the first ODD was received in Jan 2023
- At SOHO 2023, positive preclinical data demonstrated LP-284’s potent anti-tumor activity as a monotx. as well as in combination with FDA-approved lymphoma targeting antibody Rituximab in High-Grade B-cell Lymphoma (HGBL)
Mocravimod (KRP203)

- US FDA granted ODD to mocravimod for the ‘treatment to improve outcome following HSCT in hematologic malignancies’ to increase leukemia-free survival by enhancing a graft-versus-leukemia (GvL) response
- Mocravimod is currently being investigated as an adjunctive and maintenance treatment for patients with AML (n=250) receiving allogeneic HSCT in P-III (MO-TRANS) trial across the US, EU, Southeast Asia and Latin America
- The results from a P-Ib/IIa trial in patients with HM led by Piothera supported the development of mocravimod for the treatment of blood cancer and improved of CAR-T cell therapy
- The first ODD granted by the US FDA for the ‘prevention of GvHD’ in Mar 2022
THIO-101

- THIO is presently under preclinical stage and has shown positive results during in vivo analysis
- The P-II THIO-101 study is currently recruiting patients with advanced NSCLC
- This is THIO’s third ODD, drug also holds ODDs for HCC and SCLC since 2022
Tenapanor

- Xphozah (tenapanor) has been designated as an ODD by the US FDA to treat Pediatric Hyperphosphatemia. Xphozah is a single 30mg BID tablet
- The FDA approved Xphozah as an add-on medication for adults with dialysis-dependent CKD who do not respond well to phosphate binders or who cannot tolerate any dosage of phosphate binders in October 2023
AL102

- The P-II/III (RINGSIDE) trial is evaluating AL102 in patients with desmoid tumors
- AL102 is made to prevent the gamma-secretase from completing the cleavage step that is necessary for Notch activation, hence suppressing the expression of Notch gene targets
- The US FDA has also granted FTD to AL102 for the treatment of DT Ayala obtained an exclusive, worldwide license to develop and commercialize AL102 from BMS in Nov 2017
SIRPant-M (SI-101)

- SIRPant-M, an autologous SIRPαlow activated macrophage therapy, has been awarded ODD by the US FDA for the treatment of T-cell lymphoma
- SIRPant M is an autologous cancer-agnostic macrophage cell therapy manufactured using PhagoAct as a monotx. and inn combination with other immuno-stimulatory modalities such as radiotherapy and immune checkpoint inhibitors
- SIRPant-M is being assessed for the treatment of (r/r NHL, SI-101), T-cell lymphoma is a group of rare blood cancers classified under NHL and TCL is a currently uncurable form of lymphoma
NTLA-2002

- The P-I/II clinical trial evaluates the safety, tolerability, PK & PD of NTLA-2002 in patients with Type I/II HAE, includes the measurement of plasma levels of KLKB1 levels and activity, as well as HAE attack rate
- Dose selected from P-I (SAD) portion will be further evaluated in P-II, randomized, PBO-controlled study. The P-I/II study will identify dose of NTLA-2002 for use in future studies
- NTLA-2002 is an in vivo CRISPR/Cas9 genome editing designed to prevent angioedema attacks in patients with HAE as it inactivates the KLKB1 gene which encodes for prekallikrein and NTLA-2002 was also granted ODD and RMAT Designation by the US FDA, the Innovation Passport by the UK MHRA, and PRIME designation by the EMA
Rhenium (186Re) Obisbemeda

- The P-I/IIa (ReSPECT-LM) study evaluating the Rhenium (186Re) in Breast Cancer patients with LM
- Enrolment of Cohort 4 is completed, and enrolment of Cohort 5 scheduled to start by YE 2023 following standard safety review
- Plus Therapeutics has already received US FDA FTD to treat LM Updated results from ReSPECT-LM study were presented at Society for Neuro-Oncology Annual Meeting November 15-19, 2023.

KDPP Model

- The KDPP (Kidney Disease Progression Prediction) model helps to assess the risk of kidney disease progression and initiation of RRT (dialysis or transplant), within a period of 1 year, 2 years and 5 years
- AWAK & Ever Fortune.AI (EFAI, a spin-off company from CMUH based in Taiwan) has entered in a strategic partnership in Sept 2021 for the development of this predictive AI solution
- AWAK has also entered a research collaboration with Singapore General Hospital to jointly work on developing new AI models as well as testing the existing KDPP model using the hospital’s de-identified CKD patient database
KATE Sepsis

- This demonstrated the ability to improve early detection of sepsis over standard of care at ED Triage over screening protocols by up to 118%
- KATE Sepsis predictions have a higher sensitivity with a 74%, 80% & 118% improvement for sepsis, severe sepsis & septic shock
- This sensitivity improvement is achieved without a decrease in specificity, which is 95% for KATE Sepsis. These results have been published in Proprint publication
ACR-368

- ACR-368 OncoSignature assay, developed specifically to predict tumor sensitivity & it is used in ongoing registrational-intent study to treat patients. ACR-368 is being developed using proprietary Acrivon Predictive Precision Proteomics/ AP3 platform
- Acrivon has partnered with Akoya Biosciences to co-develop, validate, and commercialize Acrivon’s ACR-368 OncoSignature assay
TriVerity Acute Infection and Sepsis Test System

- FDA granted BDD to TriVerity Acute Infection and Sepsis Test System developed by Inflammatix
- TriVerity is expected to be eligible for CMS – NTAP program which will help in the adoption of tool in hospitals as it will be subsidized
- CMS may include TriVerity in TCET role with expected decision in Dec 2023 The SEPSIS-SHIELD study (NCT04094818) is necessary for 510(k) clearance of the TriVerity Test System by the FDA
- The multi-center trial has already enrolled 955 of the estimated patients (n=1,500) targeted. The company estimates FDA submission and study completion will take place in 2024

Epcoritamab

- The EMA and the US FDA granted BTD for epcoritamab (an investigational T-cell engaging bispecific AB) SC formulation, for the treatment of r/r FL after 2 or more therapies
- The results from the P-I/II (EPCORE) NHL-1 study evaluating the safety and preliminary efficacy of SC epcoritamab in (n=128) adult patients with relapsed, progressive or refractory CD20+ mature B-cell NHL incl. FL.
- Data from the FL cohort was presented at ASH 2023 Epcoritamab is being co-developed by AbbVie and Genmab
- With both sharing commercial responsibilities in the U.S. and Japan, with AbbVie has global rights Epcoritamab (brand name EPKINLY (US) & TEPKINLY (EU)) approved in adults with certain types of large B-cell lymphoma (LBCL), including DLBCL, globally
Qtorin rapamycin

- The BTD is based on positive results from P-II-multicenter, open-label, QD, QTORIN rapamycin in Microcystic LMs. After receiving the treatment for 12 wks, 100% of the patients (n =12) in the P-II study were assessed as “Much Improved” or “Very Much Improved” by the Clinician Global Impression of Change
- On the basis of positive results from P-II and meeting with FDA (after completion of P-II)
- Palvella already announced a pivotal P-III trial in ~50 adults and pediatric patients It has received FDA’s BTD, FTD, and ODD for Microcystic LMs & FTD for the prevention of BCCs in Gorlin Syndrome also received ODD from EMA for Microcystic LMs

ATSN-101

- RMAT designation was received on positive 6mos. efficacy data from the company’s ongoing P-I/II (ATSN-101) study, with expected 12mos. data by the YE’23
- The U.S FDA granted RMAT designation to ATSN-101, a gene therapy for patients with Leber congenital amaurosis caused by biallelic mutations in GUCY2D (LCA1)
- ATSN-101 also received ODD from the FDA, for the treatment of LCA1

Cell Pouch System

- The company’s Hemophilia A program has received ODD and RPDD both by the US FDA Sernova collaborating with the University of Piemonte Orientale, Italy (Antonia Follenzi MD, Ph.D. Professor of Histology and Cell and Gene therapy)
- Dr. Follenzi is a pioneer of cell and gene therapy approaches to cure Hemophilia A. Hemophilia A program and cell pouch combine with a patient’s own cells and will not require the use of immunosuppression medications. This therapy is intended to replace Factor VIII
EDG-5506

- EDG-5506 has received ODD by the US FDA for the treatment of Duchenne and Becker muscular dystrophy and RPDD for Duchenne and FDA previously granted FTD to EDG-5506 for Becker
- The P-II (CANYON) cohort study enrollment EDG-5506 evaluating safety and effects on function and biomarkers of muscle damage in Becker, with additional adult males (n=120) patients presently enrolling in pivotal cohort called GRAND CANYON
- In Duchenne, the P-II study, LYNX, assessing safety, PK and biomarkers of muscle damage, and FOX study, which includes children and adolescents previously treated with gene therapy

CSA-131

- QIDP designation grants 5 yrs. of additional market exclusivity & the potential for FTD
- N8 Medical has also developed a CSA-131 coated endotracheal tube designed to prevent VAP in mechanically ventilated ICU patients. Another study is underway at Prime Hospital in the UAE & other countries
- FDA has designated the CeraShield Endotracheal Tube as a “breakthrough device” CSA-131 is a synthetic non-peptide mimic of the endogenous antimicrobial peptide LL-37 which forms a key component of the body’s innate immune system
Related Post: New Drug Designations – October 2023


